Alzheimer’s disease research is a vital field that seeks to unravel the complexities of this devastating neurodegenerative condition. Recent studies led by neuroscientist Beth Stevens are shedding light on the crucial role of microglial cells, which function as the brain’s immune system. These immune cells are responsible for maintaining brain health by removing damaged cells and modulating neuronal connections, yet their malfunction can contribute to various neurodegenerative diseases, including Alzheimer’s. As the U.S. population ages, understanding these processes becomes increasingly urgent, especially considering that the number of Alzheimer’s cases is expected to double by 2050. Through innovative research, including new potential treatments and early detection biomarkers, Stevens’ work is paving the way for future breakthroughs in Alzheimer’s treatment.
The investigation of Alzheimer’s disease, often linked with cognitive decline in older adults, is a crucial part of ongoing scientific efforts to address neurodegenerative disorders. Researchers like Beth Stevens have focused on the brain’s immune response, primarily through microglial cells that protect and regulate neuronal health. This exploration of the immune system’s influence on the brain has revealed significant insights into conditions such as Alzheimer’s and Huntington’s disease. As we advance our understanding of these mechanisms, the development of effective therapies and diagnostic measures could revolutionize how we address cognitive impairments associated with aging. Research initiatives are becoming increasingly essential in light of projections that suggest a substantial rise in Alzheimer’s cases, emphasizing the need for innovative solutions and preventative strategies.
Understanding Microglial Cells in Alzheimer’s Disease Research
Microglial cells play a crucial role in maintaining brain health as they serve as the brain’s immune system. These cells constantly patrol the neural landscape, seeking out damaged cells and orchestrating cleanup operations. Recent research, notably by Beth Stevens, has unveiled their complex behavior, particularly in the context of neurodegenerative diseases such as Alzheimer’s. Stevens’ findings indicate that while microglia are essential for synaptic pruning and overall neural maintenance, their mismanagement can contribute to the pathogenesis of Alzheimer’s disease. This bipartite role of microglia highlights a significant area of study, aiding the quest for effective Alzheimer’s treatments.
Furthermore, the interplay between microglial dysfunction and neurodegenerative diseases reveals a potential target for intervention. As Stevens emphasizes, understanding the mechanisms behind microglial activity can lead to the development of innovative therapeutic strategies aimed at Alzheimer’s. By identifying biomarkers that reflect microglial activity, researchers can also facilitate early detection of Alzheimer’s disease, which is crucial for timely interventions that could slow disease progression.
The Role of Microglial Cells in Neurodegenerative Diseases
Neurodegenerative diseases, including Alzheimer’s, Parkison’s, and Huntington’s, often share common pathogenic mechanisms involving microglial cells. These immune cells are responsible for both neuroprotection and neurodegeneration, which places them at the forefront of research. Their ability to either promote or hinder maintaining neuronal health can have profound implications for the progression of these disorders. For example, the inappropriate pruning of synapses by hyperactivated microglia could exacerbate the cognitive decline seen in Alzheimer’s patients, making these cells a critical focus for therapeutic strategies aimed at neurodegenerative diseases.
As the scientific community delves deeper into the functions of microglia, there is an increasing demand for novel therapeutic approaches that modulate their activity. Research led by Beth Stevens has opened pathways for understanding how manipulating microglial function can alter the course of neurodegenerative diseases. By targeting the molecular pathways that govern microglial actions, there is the potential to develop drugs that can either suppress neuroinflammatory responses or enhance protective mechanisms, creating a dual-pronged approach to combatting Alzheimer’s.
Influence of Beth Stevens on Alzheimer’s Treatments
Beth Stevens’ pioneering research has positioned her as a leading figure in the search for effective Alzheimer’s treatments. Her work has significantly advanced our understanding of microglial functions and their implications for neurodegenerative diseases. With funding from federal agencies like the National Institutes of Health, Stevens has explored how microglial cells contribute to Alzheimer’s disease progression, which has substantial implications for creating targeted therapies. The insights garnered from her studies are imperative for developing medications that could help alleviate the burden of Alzheimer’s on patients and their families.
Moreover, Stevens’ emphasis on foundational research demonstrates the importance of curiosity-driven science in uncovering answers to complex questions about brain health. By illuminating the relationship between microglia and synaptic pruning, her research has helped lay the groundwork for novel therapeutic strategies that aim to enhance brain immune system functions. As the population ages and the number of Alzheimer’s cases rises, Stevens’ contributions could play a vital role in developing future interventions that not only slow the progression of the disease but enhance the quality of life for those affected.
Impact of Aging Population on Alzheimer’s Cases
As the U.S. population continues to age, the prevalence of Alzheimer’s disease is projected to escalate dramatically, with estimates suggesting a doubling of cases by 2050. This alarming trend is likely to place a tremendous strain on health care systems, with associated costs soaring from $360 million to an unimaginable $1 trillion annually. This impending crisis underscores the urgent need for comprehensive research into Alzheimer’s treatment, particularly exploring neuroinflammatory processes involving microglial cells and their role in neurodegenerative diseases.
Addressing the growing incidence of Alzheimer’s disease necessitates a multi-faceted approach. Research initiatives like those led by Beth Stevens aim not only to expand our understanding of the disease mechanism but also to develop effective therapies that can transform the management of Alzheimer’s. Investing in research that focuses on the brain’s immune system could lead to groundbreaking advancements in how we prevent, detect, and treat Alzheimer’s, ultimately mitigating the expected healthcare burden and improving outcomes for millions of patients.
The Importance of Curiosity-Driven Research in Neuroscience
Curiosity-driven research plays a pivotal role in advancing our understanding of complex health issues, particularly in neuroscience. This type of investigation allows scientists to explore unexplored territories, leading to surprising findings and potential breakthroughs. Beth Stevens’ journey in studying microglial cells exemplifies how following scientific curiosity can yield significant insights into Alzheimer’s disease and other neurodegenerative disorders. By pursuing basic science without preconceived notions of application, Stevens has set the stage for therapeutic innovations that could reshape how we approach Alzheimer’s.
Encouraging curiosity in scientific exploration is essential for fostering new ideas and methodologies in the fight against Alzheimer’s. Researchers who are granted the freedom to explore the intricacies of brain function are more likely to uncover novel therapeutic targets. This exploration often leads to collaborations that further broaden scientific horizons, combining expertise in neurology, immunology, and pharmacology, which ultimately enhances the arsenal of tools available to combat Alzheimer’s disease.
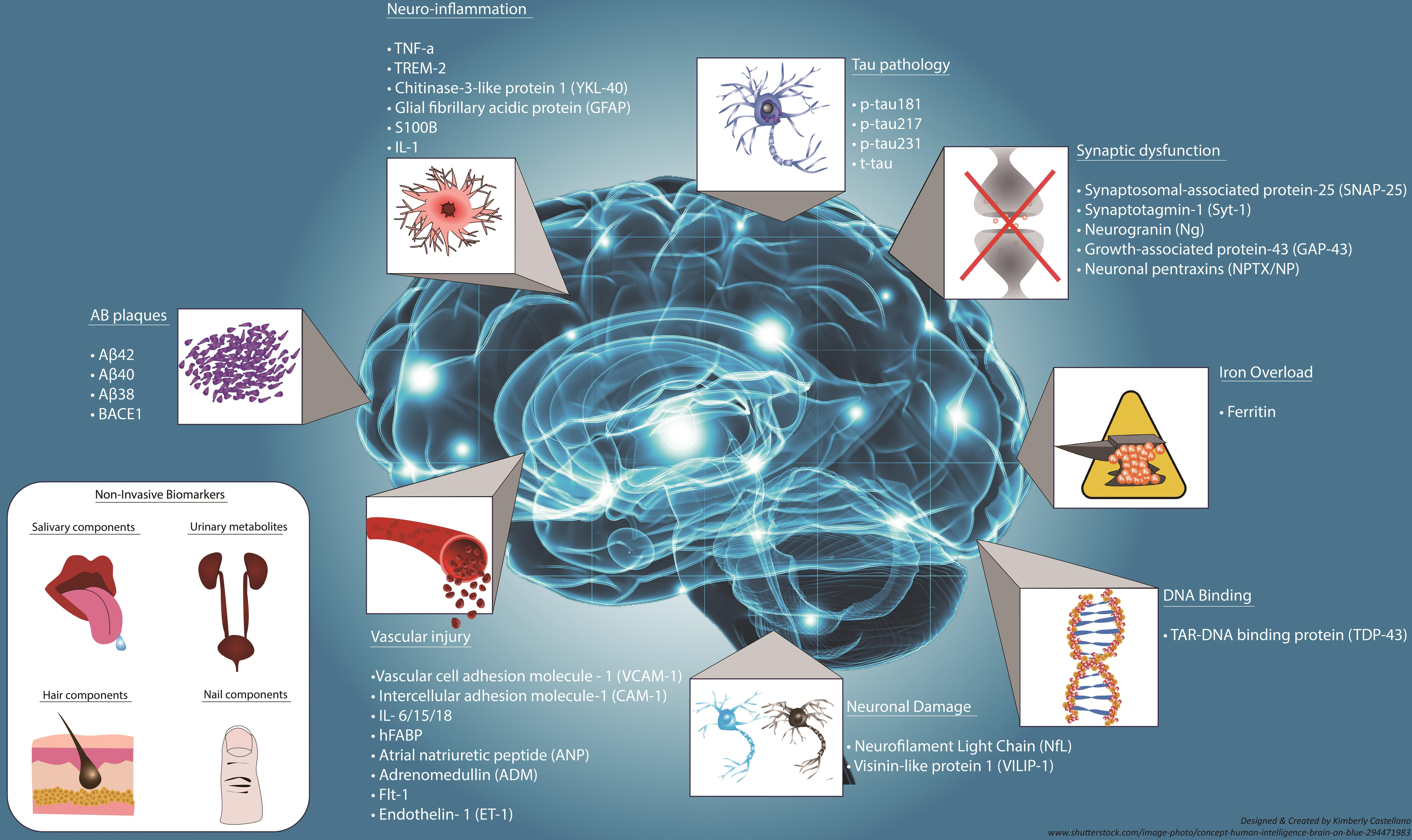
Exploring New Biomarkers for Early Alzheimer’s Detection
With Alzheimer’s disease expected to affect an increasing number of individuals, the identification of new biomarkers is critical for early diagnosis and intervention. Biomarkers derived from studies of microglial activity have the potential to revolutionize how we diagnose Alzheimer’s disease. By detecting changes in microglial functions associated with neurodegeneration, researchers like Beth Stevens aim to develop tests that can identify Alzheimer’s in its earliest stages, enabling preemptive treatment strategies that could slow disease progression and improve patient outcomes.
Implementing these new biomarkers into clinical practice poses challenges but holds the promise of significantly impacting the lives of millions at risk for Alzheimer’s. Early detection facilitates access to therapeutic measures that could delay the onset of symptoms and empower patients to maintain their independence for longer. As research continues to focus on the relationship between microglia and neurodegenerative diseases, the link between these biomarkers and specific pathological changes in the brain will enhance our ability to combat Alzheimer’s effectively.
Federal Funding: A Cornerstone for Alzheimer’s Research
Federal funding has been instrumental in advancing research on Alzheimer’s disease and related neurodegenerative disorders. Organizations like the National Institutes of Health (NIH) have provided essential resources and support for scientists like Beth Stevens, allowing them to pursue groundbreaking studies on microglial cells and their role in Alzheimer’s progression. The support for basic science is vital, as it underpins discoveries that can lead to the development of new diagnostics and therapeutics for Alzheimer’s.
In recent years, there has been a growing recognition of the need for increased federal investment in Alzheimer’s research. As the demand for effective treatments rises alongside the aging population, the government must prioritize funding for studies that investigate the mechanisms of neurodegeneration. By securing robust financial backing, researchers can continue to explore the intricacies of the brain’s immune system and contribute to the ongoing battle against Alzheimer’s disease.
The Future of Alzheimer’s Research: Innovations on the Horizon
The future of Alzheimer’s research is poised for transformative innovations, driven by advances in our understanding of microglial cells, neuroinflammation, and immune response in the brain. Researchers are currently exploring cutting-edge technologies, including imaging tools and genetic profiling, to delve deeper into the complex interplay of factors that contribute to Alzheimer’s disease. This exploration could lead to breakthroughs in treatment modalities, offering hope to millions affected by this neurodegenerative condition.
Moreover, as research progresses, there is a focus on developing personalized treatment strategies that account for the unique biological profiles of individuals diagnosed with Alzheimer’s disease. By leveraging insights about microglial function and their involvement in synaptic maintenance, the future holds promise for creating tailored interventions that utilize the brain’s immune system more effectively. Through sustained innovation and collaboration across scientific disciplines, the quest to unravel the mysteries of Alzheimer’s disease continues to gain momentum.
Frequently Asked Questions
What role do microglial cells play in Alzheimer’s disease research?
Microglial cells are crucial for maintaining brain health and function. In Alzheimer’s disease research, they act as the brain’s immune system, clearing out damaged cells and synapses. However, improper pruning by microglia can contribute to neurodegenerative diseases like Alzheimer’s, highlighting their importance in ongoing research led by scientists like Beth Stevens.
How has Beth Stevens contributed to our understanding of neurodegenerative diseases such as Alzheimer’s?
Beth Stevens has transformed Alzheimer’s disease research by investigating microglial cells and their role in the brain’s immune system. Her findings suggest that dysfunctional microglial activity can lead to abnormal synapse pruning, which is linked to neurodegenerative diseases, paving the way for new treatment options.
Why is the study of the brain immune system significant in Alzheimer’s treatment research?
Understanding the brain immune system is vital in Alzheimer’s treatment research because it sheds light on how microglial cells affect synaptic health. Insights from this research can lead to the development of therapies aimed at correcting microglial dysfunction, which could ultimately improve patient outcomes in Alzheimer’s disease.
What are the implications of improper pruning by microglial cells in Alzheimer’s disease?
Improper pruning by microglial cells can exacerbate the progression of Alzheimer’s disease and other neurodegenerative disorders. This ongoing research emphasizes the necessity of targeting microglial activity to develop effective treatment strategies for Alzheimer’s, as demonstrated by the work of Beth Stevens and her laboratory.
How can the research on microglial cells impact the future of Alzheimer’s disease diagnosis?
Research on microglial cells opens doors for identifying new biomarkers that could detect Alzheimer’s disease earlier. By understanding how these immune cells function in the brain, researchers can develop diagnostic tools that may lead to timely interventions in Alzheimer’s treatment.
What challenges does Alzheimer’s disease research face in addressing the aging population?
As the U.S. population ages, Alzheimer’s disease cases are projected to double by 2050, presenting significant challenges for research and healthcare systems. Addressing these challenges requires advances in understanding neurodegenerative diseases, like those driven by research into microglial cells, to develop effective treatments and improve care.
What foundational support is essential for advancing Alzheimer’s disease research?
Foundational support, particularly from federal funding agencies like the NIH, has been essential for advancing Alzheimer’s disease research. This funding facilitates long-term studies, such as those conducted by Beth Stevens, that explore the complex roles of microglial cells and their impact on neurodegenerative diseases.
What is the significance of ‘curiosity-driven science’ in Alzheimer’s research?
Curiosity-driven science is significant in Alzheimer’s research as it allows scientists to explore fundamental questions about brain function and disease mechanisms without immediate practical applications. This type of research has led to crucial discoveries about microglial cells and their role in neurodegenerative diseases, ultimately informing new treatment strategies.
| Key Point | Details |
|---|---|
| Research Focus | Understanding the role of microglial cells in Alzheimer’s disease and other neurodegenerative diseases. |
| Importance of Microglia | Microglia serve as the immune system of the brain, facilitating the removal of damaged cells and pruning synapses. |
| Implications of Findings | Improper pruning by microglia may contribute to the progression of Alzheimer’s disease and provide insights for new treatments. |
| Funding and Support | Research funded largely by the National Institutes of Health and other federal sources to explore foundational science. |
| Future of Alzheimer’s Treatment | Stevens’ work could lead to new medications and biomarkers for early detection, significantly affecting millions. |
| Rising Dementia Cases | The number of Alzheimer’s cases in the U.S. is projected to double by 2050, increasing the treatment costs significantly. |
Summary
Alzheimer’s disease research is continuously evolving, with groundbreaking discoveries like those made by Beth Stevens radically transforming our understanding of brain immunity and dysfunction. By investigating the role of microglial cells, Stevens is paving the way for potential treatments that may mitigate symptoms and improve the quality of life for millions of individuals affected by Alzheimer’s. As researchers continue to unravel the complexities of neurodegenerative diseases, the hope is that innovative therapies and early detection biomarkers will emerge, ultimately leading to a brighter future for Alzheimer’s patients.
Comments are closed, but trackbacks and pingbacks are open.