Microglial research is at the forefront of advancements in understanding the brain’s immune system, particularly in relation to neurodegenerative diseases such as Alzheimer’s disease. These specialized cells play a crucial role in maintaining brain health by patrolling for cellular debris and aiding in synaptic pruning, which is essential for proper neural communication. However, recent findings suggest that dysregulation of microglial activity can lead to detrimental effects, contributing to conditions like Alzheimer’s and Huntington’s disease. Researchers, including Beth Stevens, are discovering new biomarkers for Alzheimer’s that stem from these critical observations, paving the way for innovative treatments and improved patient care. As the number of Americans living with Alzheimer’s rises, this research sheds light on how we can better protect and preserve cognitive health.
Investigating the functions of microglia provides essential insights into the broader context of brain health and immune interactions within the central nervous system. These brain-resident immune cells not only participate in synaptic remodeling during typical development but have also been implicated in various neurodegenerative conditions, including Alzheimer’s disease. The evolving understanding of the brain’s immune landscape opens avenues for novel therapeutic strategies and biomarker discovery that could revolutionize how we approach brain disorders. Pioneers in the field, like Beth Stevens, are unlocking critical information about the pathology of these diseases, which can lead to groundbreaking treatment options. As research delves deeper, it emphasizes the significance of microglial function in safeguarding against neurological decline.
The Role of Microglial Research in Understanding Alzheimer’s Disease
Microglial research has emerged as a groundbreaking field in the study of Alzheimer’s disease. These brain-resident immune cells are crucial for maintaining the health of the central nervous system. By constantly surveying the brain environment, microglia play a vital role in synaptic pruning, which is essential for neural network development and function. However, in the context of neurodegenerative diseases, including Alzheimer’s, the functioning of microglia can become maladaptive. Aberrant synaptic pruning by hyperactive microglia can lead to the loss of crucial neural connections, exacerbating cognitive decline in Alzheimer’s patients.
Recent findings from studies led by Beth Stevens and her team have highlighted the importance of these immune cells in disease progression. The Stevens Lab has focused on elucidating the signaling pathways and mechanisms that govern microglial behavior in Alzheimer’s disease. This research has not only enhanced our understanding of the basic biology of these cells but has also opened new avenues for identifying biomarkers for Alzheimer’s that could help in early diagnosis and therapy. Innovations in this field could lead to treatments that modulate microglial activity, aiming to restore healthy brain function.
Understanding the Brain Immune System and Its Implications
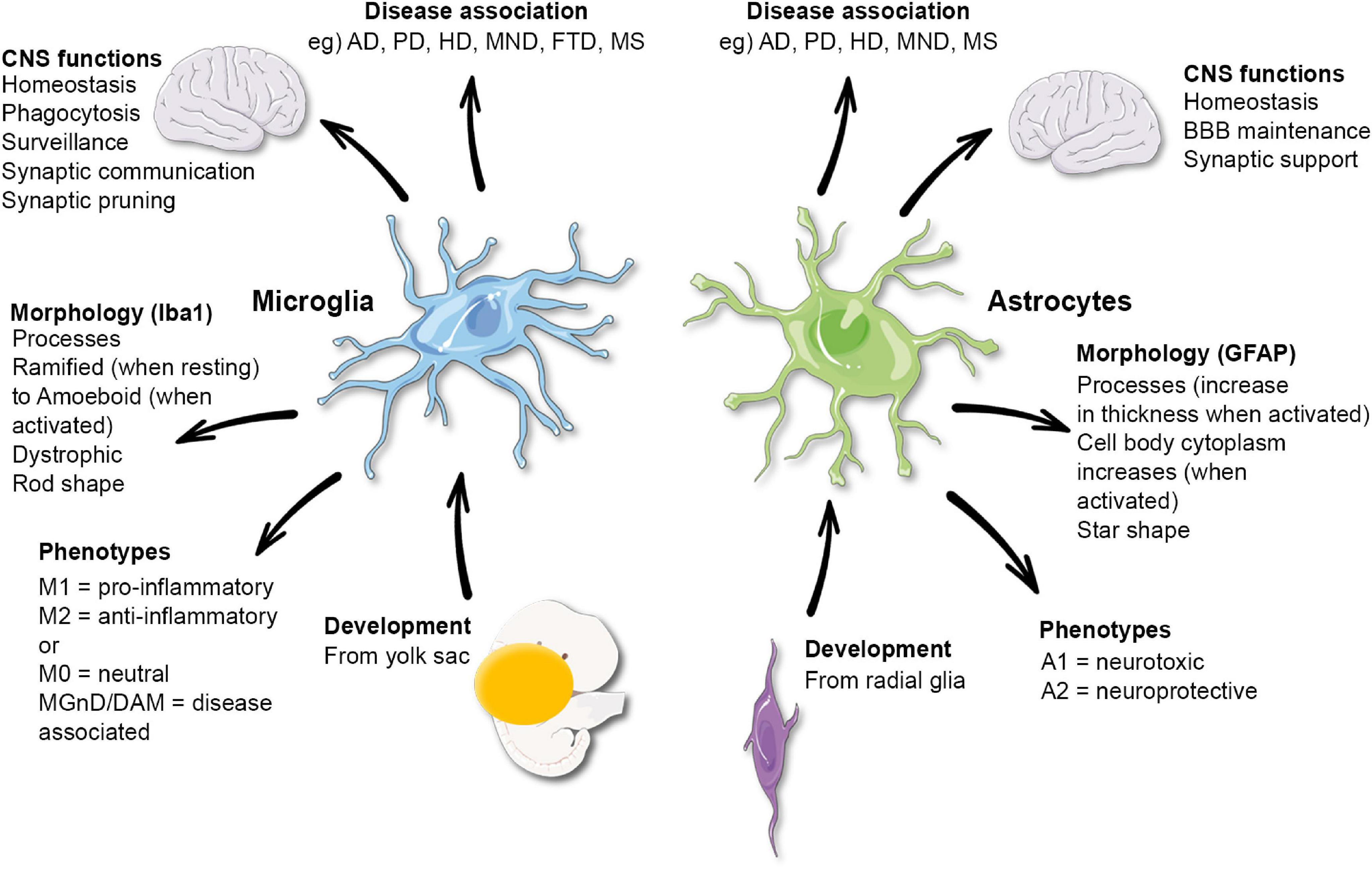
The brain’s immune system, primarily composed of microglia, plays an essential role in maintaining neuronal health and facilitating effective communication between brain cells. Microglia help regulate inflammation, clear debris from damaged neurons, and support synaptic remodeling through pruning. This function is critical for brain plasticity, ensuring that neural tissue adapts to new information and experiences. However, in neurodegenerative diseases like Alzheimer’s, dysregulation of microglial activity can lead to detrimental outcomes, including increased inflammation and neuronal death.
Current research emphasizes the need for a deeper understanding of the brain immune system to develop therapeutic strategies against neurodegenerative diseases. By targeting microglial functions, scientists hope to manipulate their activity to reduce synaptic loss and enhance neuronal survival. The ongoing studies are crucial not only for identifying potential biomarkers for Alzheimer’s but also for testing novel pharmacological agents that could modify the course of the disease, ultimately improving quality of life for millions of affected individuals.
Synaptic Pruning and Neurodegeneration
Synaptic pruning is a critical developmental process wherein excess synapses in the brain are eliminated to refine neural circuits. While this process is essential for normal brain function, its dysregulation has been implicated in various neurodegenerative diseases, including Alzheimer’s. In healthy brains, microglia act as custodians, removing weak or redundant synapses to enhance overall cognitive efficiency. Nevertheless, when microglial functions go awry—due to genetic predispositions, environmental factors, or age—abnormal pruning can lead to the destabilization of neural networks and contribute to the symptoms observed in Alzheimer’s patients.
Beth Stevens’ research into synaptic pruning investigates how microglial cells misinterpret signals in the Alzheimer’s brain, leading to excessive removal of synapses. This phenomenon not only emphasizes the delicate balance required for proper synaptic health but also highlights the potential for therapeutic intervention. By understanding the mechanisms regulating microglial pruning activity, researchers can develop strategies that might restore correct synaptic pruning processes and mitigate the cognitive decline associated with neurodegeneration.
Exploring Biomarkers for Alzheimer’s Disease
Biomarkers are crucial in the early detection and treatment of Alzheimer’s disease. They serve as biological indicators of the disease’s presence and progression, allowing for earlier, more effective interventions. Recent research has begun to unearth microglial-related biomarkers by examining the altered behavior of these cells in the Alzheimer’s brain. Stevens’ work suggests that specific microglial activation states might correlate with different stages of neurodegeneration, leading to innovative diagnostic tools that could reshape how Alzheimer’s is identified and treated.
The quest for reliable biomarkers encompasses both biological measurements and imaging techniques that can visualize changes at the molecular level. By combining insights from neuroimaging and microglial function, researchers aim to establish a comprehensive profile of Alzheimer’s progression. This integrated approach could lead to personalized treatment options, optimizing therapeutic strategies based on individual biomarker profiles and improving patient outcomes.
The Future of Neurodegenerative Disease Research
The future of neurodegenerative disease research is bright, especially with the advancements in understanding the role of microglia in Alzheimer’s disease. As researchers like Beth Stevens continue to investigate the interplay between immune responses and neural health, there is hope that new therapeutic strategies can be developed to halt or even reverse the effects of neurodegeneration. These insights are crucial as the incidence of Alzheimer’s disease is on the rise, with millions globally affected by this devastating condition.
Furthermore, interdisciplinary collaboration will be essential in pushing the boundaries of current knowledge. By integrating insights from neurobiology, immunology, and pharmacology, the research community can develop more nuanced treatments that address the multifaceted nature of Alzheimer’s disease. The ongoing exploration into the connections between microglial function, neural health, and disease progression will undoubtedly pave the way for groundbreaking interventions that could transform care for patients and improve their quality of life.
Translating Basic Science to Clinical Practice
Translating basic science into clinical practice is a significant challenge in biomedical research, particularly in fields like Alzheimer’s disease, which entail complex biological interactions. Beth Stevens emphasizes the importance of foundational research, stating that it lays the groundwork for future treatments. Initially focused on understanding the role of microglia in the visual system of mice, her groundbreaking discoveries reveal the potential implications for human health and neurodegenerative disease treatment.
This translation process requires rigorous testing and validation of findings from laboratory studies to clinical applications. It involves not only the discovery of new biomarkers and therapeutic targets but also the development of clinical trials that can evaluate the safety and efficacy of novel treatments. Engaging patients in these studies is crucial to ensuring that the insights gained from microglial research culminate in tangible benefits for those living with Alzheimer’s and related disorders.
Innovations in Alzheimer’s Drug Development
As our understanding of Alzheimer’s disease deepens, so too do the strategies for developing innovative drugs. The focus on microglial biology has led to promising avenues for drug discovery. By targeting specific pathways associated with microglial activation and synaptic pruning, researchers hope to develop drugs that can not only slow disease progression but also enhance cognitive function. The pharmaceutical landscape for Alzheimer’s is evolving, with a shift towards more precise, mechanism-based therapies rather than broadly acting symptomatic treatments.
Collaboration between academia and the pharmaceutical industry plays a crucial role in this innovative drug development process. By leveraging findings from cutting-edge microglial research, pharmaceutical companies can create a pipeline of drugs that address the underlying causes of Alzheimer’s disease. The potential for new medications that improve disease-modifying outcomes is on the horizon, and with continued investment in research, we may finally see effective treatments for this challenging neurodegenerative disorder.
The Importance of NIH Funding in Neuroscience
National Institutes of Health (NIH) funding has been pivotal in advancing our understanding of Alzheimer’s disease and microglial research. This funding supports foundational research that explores the complexities of the brain immune system, facilitating discoveries that can be translated into impactful clinical applications. For researchers like Beth Stevens, the backing from NIH not only legitimizes their work but also provides them with the resources needed to tackle ambitious projects that could lead to new treatments for Alzheimer’s and other neurodegenerative diseases.
Investments in neuroscience research from NIH have far-reaching implications, fostering a culture of innovation and sustained exploration. As neuroscience continues to evolve, ongoing support is necessary to ensure that researchers can delve into the intricacies of brain health and disease. This funding catalyzes collaboration across disciplines, enabling teams to address the multifaceted nature of neurodegenerative disorders effectively and develop solutions that benefit public health.
Engaging Public Awareness for Alzheimer’s Research
Raising public awareness about Alzheimer’s disease and the significance of ongoing research is crucial for mobilizing support and enhancing understanding of this complex condition. Efforts to engage communities in discussions about neurodegenerative diseases help demystify the challenges faced by patients and families. Highlighting groundbreaking microglial research and its implications for treatment can inspire collective action, bringing renewed focus to the need for funding and investment in scientific discovery.
Public engagement also plays a critical role in increasing participation in clinical trials, which is essential for developing new Alzheimer’s treatments. By informing the public about the importance of research participation and the potential benefits of emerging therapies, advocates for Alzheimer’s research can help bridge the gap between scientific progress and real-world applications. In doing so, they empower individuals and families affected by Alzheimer’s, reinforcing that progress in research directly impacts their lives.
Frequently Asked Questions
What role do microglial cells play in Alzheimer’s disease?
Microglial cells function as the brain’s immune system and play a crucial role in Alzheimer’s disease by clearing out dead or damaged neurons and participating in synaptic pruning. Research indicates that abnormal microglial activity can contribute to neurodegenerative diseases, including Alzheimer’s, as they may mistakenly prune healthy synapses, which disrupts neuronal communication.
How does microglial research contribute to understanding neurodegenerative diseases?
Microglial research provides insights into the immune mechanisms of the brain, revealing how these cells are involved in neurodegenerative diseases like Alzheimer’s disease. Understanding their function and behavior helps scientists identify potential biomarkers for Alzheimer’s and develop therapeutic strategies aimed at correcting synaptic pruning errors caused by microglia.
What are some potential biomarkers for Alzheimer’s disease derived from microglial research?
Recent findings in microglial research have led to the identification of potential biomarkers for Alzheimer’s disease, focusing on the inflammatory responses and synaptic pruning activities of microglia. These biomarkers can help in early detection and monitoring of the disease, facilitating timely interventions for those affected.
What implications does microglial research have for Alzheimer’s treatment?
Microglial research has significant implications for Alzheimer’s treatment by uncovering new therapeutic targets. By understanding how microglia contribute to synaptic pruning and neuroinflammation, scientists can develop drugs that modulate these processes, potentially slowing the progression of Alzheimer’s disease and improving the quality of life for patients.
How does synaptic pruning by microglia affect brain health?
Synaptic pruning by microglia is vital for maintaining brain health, as it helps refine neural circuits during development and in response to injury. However, dysregulated synaptic pruning, often observed in Alzheimer’s disease and other neurodegenerative disorders, can lead to cognitive decline and impaired brain function, underscoring the importance of microglial research in understanding these critical processes.
Why is basic research important for advancements in microglial research related to Alzheimer’s disease?
Basic research lays the groundwork for advancements in microglial research, providing foundational knowledge about how these cells operate within the brain’s immune system. This understanding is essential for translating scientific discoveries into clinical applications, such as identifying biomarkers for Alzheimer’s disease and developing innovative treatments based on microglial function.
What challenges are faced in microglial research concerning neurodegenerative diseases?
One of the primary challenges in microglial research related to neurodegenerative diseases like Alzheimer’s is understanding the complex dual role of microglia as both protectors and potential contributors to neuronal damage. Additionally, translating findings from animal studies into human applications poses significant obstacles, requiring continuous funding and support for ongoing research.
How can microglial research impact the lives of people with Alzheimer’s disease?
Microglial research can substantially impact the lives of people with Alzheimer’s disease by paving the way for new diagnostic methods and treatments. By improving our understanding of microglial functions and their role in disease progression, researchers can develop strategies that target and modify these immune responses, ultimately aiming to enhance patient care and slow the disease’s progression.
| Key Points | Details |
|---|---|
| Role of Microglia | Microglia act as the brain’s immune cells, clearing damaged cells and pruning synapses. |
| Impact on Neurodegenerative Diseases | Aberrant microglial pruning is linked to Alzheimer’s, Huntington’s, and other disorders. |
| Research Funding | Beth Stevens’ research is heavily supported by NIH funding, emphasizing the value of basic scientific research. |
| Advancements | Research lays groundwork for new biomarkers and treatments for neurodegenerative diseases like Alzheimer’s. |
| Importance of Basic Science | Basic science fosters curiosity and leads to breakthroughs that can transform understanding and treatment of diseases. |
| Personal Journey | Stevens highlights the unpredictable path of research and the importance of following scientific curiosity. |
Summary
Microglial research plays a crucial role in understanding neurodegenerative diseases and the brain’s immune system. Beth Stevens’ groundbreaking work has shifted perspectives on microglia, revealing their significant involvement in conditions like Alzheimer’s disease. The ongoing studies not only illuminate the functions of microglial cells but also pave the way for novel treatments that could transform lives. The investment in basic science remains essential for unraveling the complexities of brain health and developing effective therapeutics for millions affected by these debilitating diseases.
Comments are closed, but trackbacks and pingbacks are open.